
Newsroom
회사, 제품, 이벤트 및 업계 뉴스/보도자료
AGC Biologics Expands Cell and Gene Therapy CDMO Capacity at its U.S. site
- Responding to increased demand for viral vectors -
AGC (AGC Inc., Headquarters: Tokyo, President: Yoshinori Hirai), a world-leading manufacturer of glass, chemicals, high-tech materials, has decided to expand the manufacturing capacity of its biopharmaceutical CDMO*1 business subsidiary, AGC Biologics, Inc. (Headquarters: United States), for cell and gene therapy applications. In order to meet strong demand, AGC Biologics will increase the capacity of its virus vector*2 manufacturing facility at its Longmont, Colorado, U.S. site. The new facility is scheduled to start operation in the third quarter of 2022*3.

Longmont site of AGC Biologics in the U.S.
The cell and gene therapy field is developing rapidly and is expected to grow at an annual rate of more than 30%*4. In order to ensure manufacturing capacity in the U.S., which is the world's largest market for gene and cell therapy, AGC acquired this site from Novartis in August 2021, and has been expanding its CDMO services in the gene and cell therapy field.
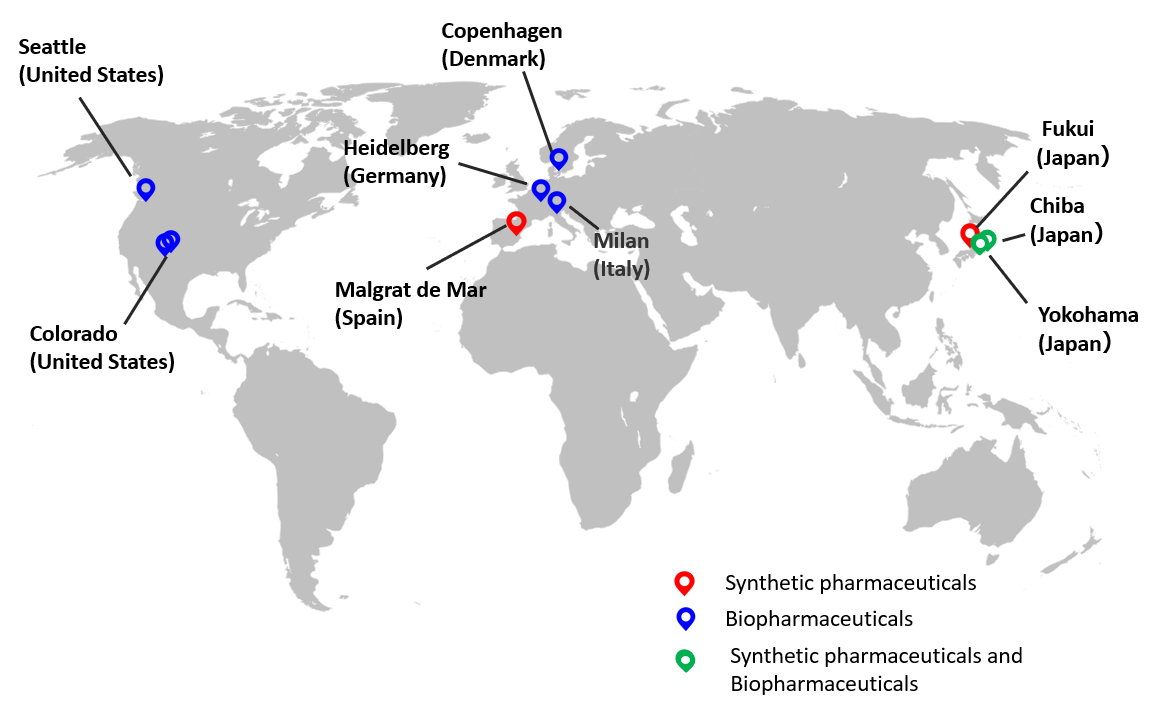
This expansion will include the introduction of a new suspension facility*5 suitable for the mass production of viral vectors, which is expected to grow rapidly in the field of gene and cell therapy. This expansion will be carried out with technical support from the Milan (Italy) site, which is also a cell and gene therapy site and has pioneered the introduction of floating culture facilities through the capacity expansion which was already announced. In collaboration with the Heidelberg (Germany) site, which is engaged in the contract manufacturing of plasmid DNA, a raw material for gene and cell therapy, we will continue to provide a one-stop global CDMO service from plasmids to gene and cell therapy drugs.
Under its medium-term management plan AGC plus-2023, the AGC Group has positioned its life science business, including biopharmaceutical CDMOs, as one of its strategic businesses and has aggressively made acquisitions and capital investments to expand this business. The Company aims to expand this business from 44.9 billion yen in 2018 to 135.0 billion yen in 2022 and to over 200 billion yen in 2025. AGC Group will work to provide its customers in each region with globally unified, high-quality services, contributing to the well-being of patients and the wider society as a whole.
Notes
*1 CDMO: Contract Development & Manufacturing Organization. A company which is contracted on behalf of another company to provide product manufacturing services as well as the development of manufacturing processes.Synthetic pharmaceuticals: Pharmaceuticals manufactured through chemical synthesis, small molecule pharmaceuticals.
*2 Viral vector: A vector is used to carry the target gene into a cell. Among vectors, viral vectors are those using modified cultured viruses.
*3 Fiscal year of the Company: from January 1 to December 31
*4 30% or higher annual growth: CAGR from 2020 to 2024. AGC estimate based on EvaluatePharma® World Preview 2017, Outlook to 2022 and other sources.
*5 Suspension facility: A culture method used in the manufacture of viral vectors in which cells are free-floating in the culture media. It enables culturing on a larger scale compared with the adherent method, in which cells are attached to a substrate.
Reference
â– AGC Group’s CDMO business locations
Media inquiries
Chikako Ogawa, General Manager, Corporate Communications & Investor Relations Division
AGC Inc.
Contact: Nakao
TEL: +81-3-3218-5603
E-mail: info-pr@agc.com
Personal information is handled in accordance with our Privacy Policy
